From Dormant to Dysfunctional: Mapping the Aging Trajectory of Stem Cells
A groundbreaking review published in Cell Stem Cell introduces a bold new framework to understand how somatic stem cells age—and why their regenerative abilities decline over time.
Authored by leading researchers Thomas A. Rando (UCLA), Anne Brunet (Stanford), and Margaret A. Goodell (Baylor College of Medicine), the study highlights five core functional hallmarks that define the aging trajectory of adult stem cells.
Rather than focusing on general cellular damage, this new paradigm emphasizes the behavior and fate of aging stem cells across multiple tissues, from blood to brain.
It’s not just about damaged DNA or faulty mitochondria,” says Brunet. “It’s about how stem cells behave—how deeply they sleep, how resilient they are, and whether they can still make the right decisions.”
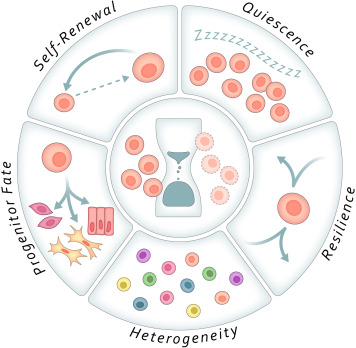
The Five Hallmarks of Stem Cell Aging:
- Depth of Quiescence
With age, stem cells may become too deeply dormant to respond effectively—or too shallowly quiescent, leading to premature exhaustion.
- Self-Renewal Propensity
The ability to maintain a healthy stem cell pool falters, with either excessive self-renewal and poor differentiation, or outright loss of stem cell numbers.
- Fate of Progeny
Aged stem cells often produce the wrong types of cells—such as more astrocytes and fewer neurons, or fibrogenic instead of myogenic cells—leading to tissue dysfunction.
- Resilience
Stem cells in older organisms show increased vulnerability to stress, injury, and death, impairing regeneration when it’s needed most.
- Population Heterogeneity
Stem cell pools lose their diversity with age, becoming dominated by dysfunctional or mutated clones that limit adaptability and repair.
“These changes aren’t just side effects of aging—they’re central to why tissues stop healing,” says co-author Dr. Rando. “If we can restore these functions, we might restore regenerative capacity itself.”
As life expectancy rises, understanding the limits of tissue regeneration becomes a medical priority. This new model doesn’t just explain stem cell aging—it reveals actionable targets to slow or reverse it.
Full study available at: Cell Stem Cell, July 2025 – DOI: 10.1016/j.stem.2025.06.004
Share this with your circle