Pausing Death: Scientists Reframe Necrosis as a Treatable Target in Aging and Chronic Disease
Is death always irreversible? A new review suggests it might not have to be.
In a bold and thought-provoking article published in Oncogene on May 29, 2025, an international team of researchers led by Dr. Carina Kern (UCL) has called for a paradigm shift in how we understand—and potentially treat—necrosis, a form of catastrophic cell death long considered beyond intervention.
The review, titled “Necrosis as a disruptor of cellular resilience across organs and lifespan,” offers a sweeping analysis of necrosis as a central, yet overlooked, driver of organ damage, aging, and inflammatory disease. Drawing from cellular biology, clinical observations, and even space medicine, the authors argue that the key to preserving tissue function and extending healthspan may lie in targeting how we die at the cellular level.
What Is Necrosis—and Why Now?
Unlike apoptosis, the tidy, programmed form of cell death that serves as a natural mechanism for tissue renewal and immune defense, necrosis is uncontrolled and violent. Cells undergoing necrosis swell, rupture, and release inflammatory substances into surrounding tissue—causing what the authors call “a chain reaction of dysfunction.”
Historically, necrosis was considered an unavoidable outcome of extreme stress—trauma, stroke, toxins, infection. But recent findings suggest it may also occur in slow-motion within organs affected by chronic stress and age-related decline. This “silent necrosis,” the authors argue, gradually chips away at tissue resilience.
“We tend to think of necrosis as something that happens in emergencies, but evidence is mounting that it may be silently shaping our aging process and the outcome of chronic diseases,” explains lead author Dr. Kern.
Calcium Overload and the Breaking Point
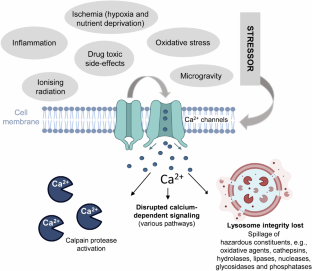
Central to the paper is the role of calcium dysregulation. Under normal conditions, calcium acts as a messenger within cells. But when too much calcium floods into the cytosol—triggered by toxins, mitochondrial failure, or ischemia—it becomes a death signal. This influx activates destructive enzymes, damages membranes, and ultimately causes the cell to rupture.
The review maps out how calcium-triggered necrosis may contribute to disease across organs:
• In the heart, it may amplify damage during heart attacks and reperfusion injury.
• In the kidneys, it may accelerate the decline associated with age and hypertension.
• In the liver and pancreas, it could mediate inflammation in conditions like NASH and diabetes.
• In the brain, necrosis may worsen outcomes in stroke and neurodegenerative diseases.
From Earth to Space: A Universal Mechanism
Perhaps most intriguingly, the authors link necrosis to space biology. Co-authors affiliated with NASA’s Translational Research Institute for Space Health (TRISH) point to necrosis-like damage observed in astronauts exposed to cosmic radiation and microgravity. In these extreme environments, the cellular stressors mirror those of accelerated aging.
“Whether on Earth or in orbit, necrosis may be a final common pathway through which stress causes organ failure,” notes Dr. Keith Siew, nephrologist and co-author from the UCL Centre for Kidney and Bladder Health.
This universal aspect makes necrosis an appealing target—not just for acute injuries but for slowing systemic degeneration.
A New Therapeutic Frontier
The authors propose a radical idea: that we should pause, block, or modulate necrosis therapeutically. This could involve:
• Inhibiting calcium entry into stressed cells.
• Targeting mitochondrial dysfunction and restoring energy homeostasis.
• Using anti-inflammatory drugs to neutralize the tissue-damaging aftermath of necrosis.
• Repurposing existing agents (e.g., calcium channel blockers, mitochondrial stabilizers) in novel ways.
Some early-stage trials and animal studies support the feasibility of this strategy, but clinical translation remains in its infancy.
Still, the paper outlines realistic opportunities—such as protecting organs before surgery, reducing chemotherapy toxicity, or managing organ transplantation injuries.
Implications Across Medicine
If necrosis can be regulated, the impact could be enormous:
• In cardiology, it could reduce infarct size and improve heart failure outcomes.
• In nephrology, it might preserve kidney function in aging populations.
• In neuroscience, it offers a new angle on neuroinflammation and synaptic loss.
• In gerontology, it aligns with the emerging view of aging as a “controlled breakdown” that could be interrupted.
Conclusion: Time to Rethink Cellular Death
Necrosis is no longer just a pathological footnote. This comprehensive review reframes it as a biological disruptor with vast relevance across the lifespan and across medical disciplines.
“We’ve spent decades studying how cells live. It’s time we study how they die—and how we might change that,” writes Kern.
By shifting the narrative from inevitable destruction to modifiable process, this paper lays the groundwork for a new era of regenerative and preventative medicine. One in which preserving life means learning how to pause death itself.
Reference
Kern C, Siew K, et al. Necrosis as a disruptor of cellular resilience across organs and lifespan. Oncogene (2025).
https://doi.org/10.1038/s41388-025-03431-y
Share this with your circle