Protein That Regulates Cellular Aging Could Open Pathways for Rejuvenation Therapies
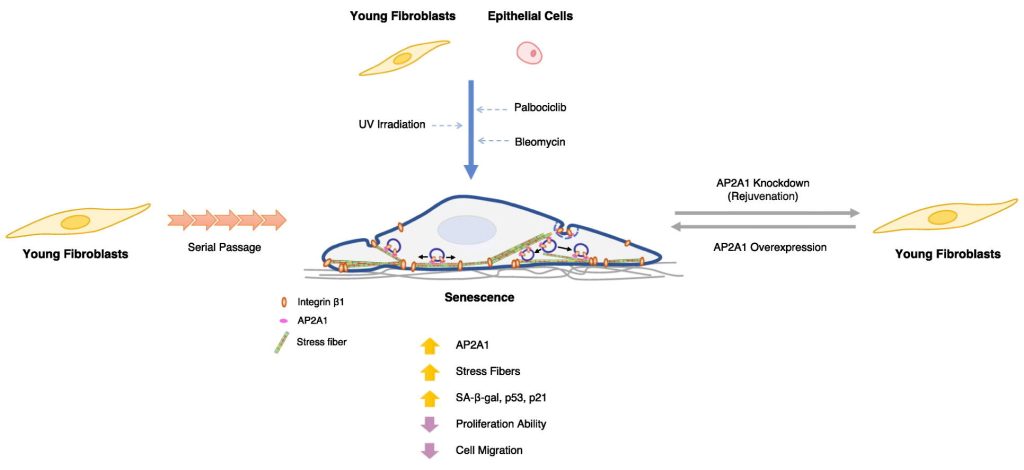
Researchers from Osaka University have identified a key protein, AP2A1 (Adaptor Protein Complex 2, Alpha 1 Subunit), that plays a critical role in regulating the transition between “young” and “old” cell states, offering potential pathways for reversing cellular aging. The study, published in Cellular Signaling, explores how AP2A1 influences the behavior of senescent cells, which accumulate with age and are associated with age-related diseases.
Senescent cells are larger than younger cells and exhibit altered stress fibers, structural elements crucial for cell movement and interaction with the environment. In this study, the researchers focused on the role of AP2A1, which is upregulated in the stress fibers of senescent cells, including fibroblasts and epithelial cells. By suppressing AP2A1 in older cells and overexpressing it in young cells, they observed significant changes in cell behavior.
Suppressing AP2A1 in older cells reversed signs of senescence and promoted rejuvenation, while overexpressing it in young cells accelerated senescence. These findings suggest that manipulating AP2A1 could be a way to influence the aging process in cells.
The researchers also found that AP2A1 closely associates with integrin β1, a protein that helps cells attach to the extracellular matrix. This interaction plays a role in strengthening cell-substrate adhesions, which may explain the larger size and altered structure of senescent cells.
By identifying AP2A1 as a key factor in cellular aging, this research opens up new possibilities for using it as a biomarker and therapeutic target for age-related diseases. The findings provide valuable insights into potential strategies for rejuvenating aged cells and developing treatments to combat the effects of aging.
Image Credits: Pirawan Chantachotikul, Shiyou Liu, Kana Furukawa, Shinji Deguchi, Cellular Signalling, Volume 127, 2025
Share this with your circle