Intestinal Mitochondrial Enzyme Disruption: A Key to Longevity?
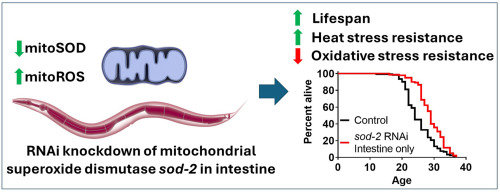
A new study published in Free Radical Biology and Medicine has revealed that selectively disrupting the mitochondrial superoxide dismutase gene sod-2 in the intestine can significantly extend lifespan. Conducted by Thomas Liontis, Jeremy M. Van Raamsdonk, and colleagues from McGill University (Canada), the research provides new insights into the complex role of reactive oxygen species (ROS) in aging and longevity.
The Role of Mitochondria and ROS in Aging
Mitochondria are key cellular organelles responsible for energy production, and they generate ROS as byproducts of metabolism. While excessive ROS levels can cause cellular damage, they also serve as important signaling molecules that regulate lifespan. The enzyme superoxide dismutase (SOD) plays a crucial role in controlling ROS levels by converting superoxide radicals into hydrogen peroxide. Intriguingly, previous studies have shown that a global disruption of sod-2 leads to increased lifespan. However, the specific tissue in which this disruption drives longevity was previously unknown.
Key Findings, Implications for Longevity Research & Future Directions
In this new study, researchers investigated the tissue-specific effects of sod-2 disruption and found that knocking down sod-2 exclusively in the intestine was sufficient to extend lifespan. Furthermore, re-expressing sod-2 in individual tissues did not prevent the longevity benefits observed in organisms with global sod-2 deletion. The findings suggest that intestinal mitochondrial superoxide dismutase plays a unique role in aging, where its suppression paradoxically promotes longevity.
Additionally, intestine-specific sod-2 knockdown was associated with increased resistance to heat stress while simultaneously reducing resistance to oxidative stress, further highlighting the complex interplay between ROS and cellular adaptation mechanisms.
These findings provide a deeper understanding of the conditions necessary for mitochondrial superoxide dismutase disruption to enhance longevity. They also open avenues for further research into targeted mitochondrial interventions that may promote healthy aging.
Stay tuned for more updates on the latest advances in aging research at the Targeting Longevity Congress 2025, to be held on October 29-30 in Berlin, Germany.
Image Credits: Thomas Liontis et al. Free Radical Biology and Medicine, Volume 229
Share this with your circle